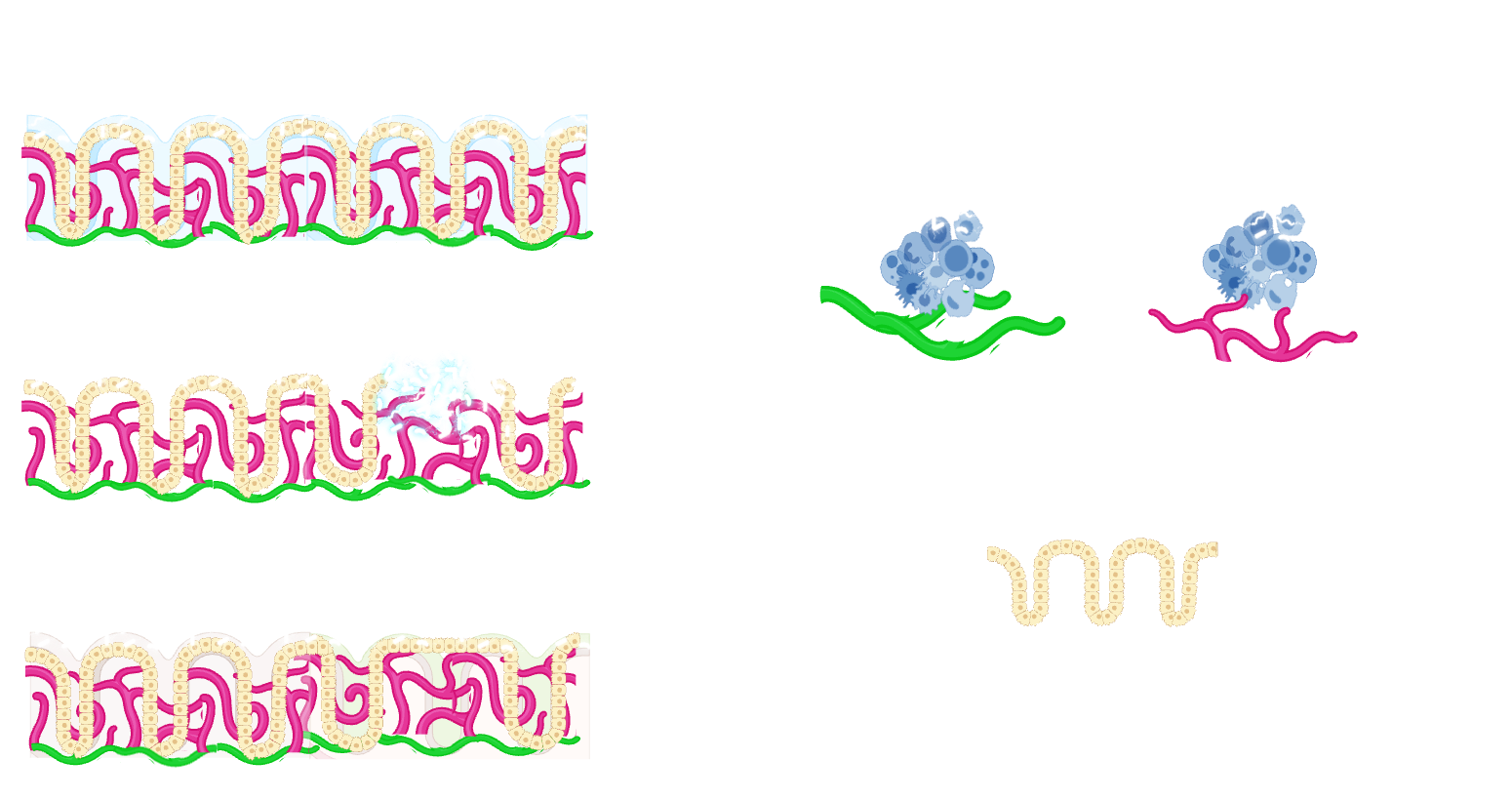
The role of blood and lymphatic vessels in intestinal regeneration and recovery
The significance of the endothelial cell niche in regeneration has recently gained recognition across various tissues. However, within the intestinal context, the role of endothelial cells has been vastly overlooked, partly due to research emphasis on other niche cells like fibroblasts and historical limitations in transcriptional and genetic tools. Therefore, our lab focuses on investigating the contribution of endothelial cells and endothelial cell-derived paracrine factors in intestinal regeneration focusing on gastrointestinal diseases, such as inflammatory bowel diseases. We aim to create a detailed, spatially, and transcriptionally resolved map of blood and lymphatic endothelial cells in inflammatory bowel diseases, using both human tissue samples and mouse models. Additionally, our work will explore immune-endothelial cell interactions to unravel the role of the endothelial cell niche in intestinal regeneration and repair
Why is this important?
Ultimately, our research will decipher the role of endothelial cells in intestinal regeneration and repair, with a focus on immune-endothelial cell interactions, advancing our understanding of gastrointestinal diseases and leading to potential new druggable targets.
How do we address this?
To achieve these goals, we employ single-cell RNA sequencing, spectral flow cytometry, spatial transcriptomics, microbiome manipulation, high-resolution imaging, and in vitro organoid systems.
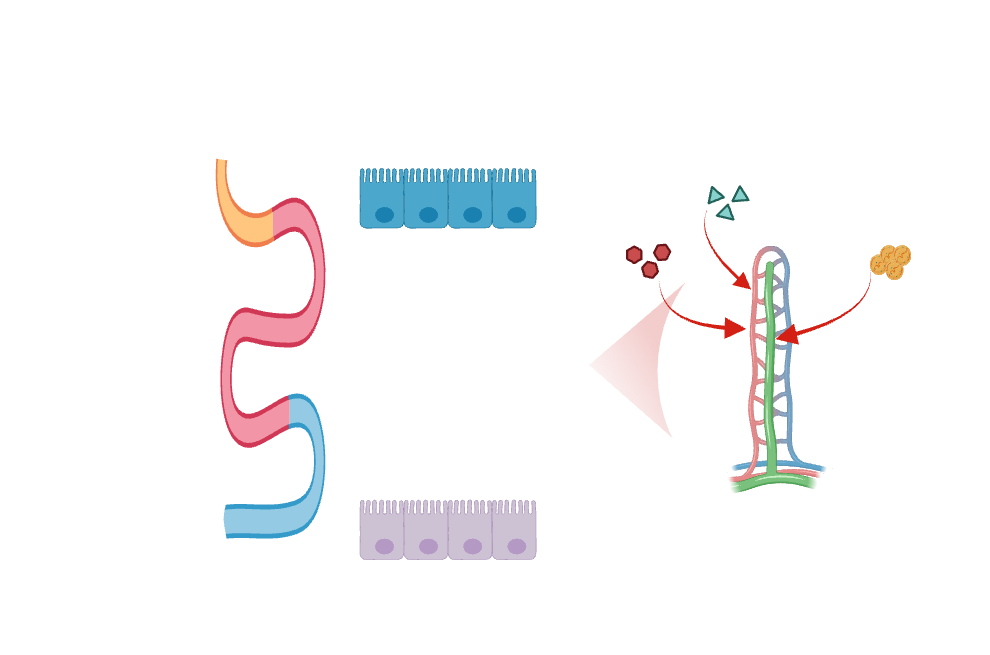
Deciphering endothelial cell regionalization along the intestine
Endothelial cells demonstrate significant diversity in their transcriptional signatures and functions across various tissues. Recent breakthroughs in single-cell RNA sequencing have unveiled an additional layer of intra-tissue diversity within endothelial cells. In the small intestine, nutrient absorption predominantly mediated by epithelial cells, is followed by transversion either through blood or lymphatic vessels. Most macromolecules are transported through blood capillaries, except for dietary lipids, which are packaged into chylomicrons and absorbed in lacteals in the small intestine. Notably, nutrient absorption and immune and microbiome composition is not uniform across the length of the intestine. Despite our growing understanding of epithelial cell zonation along the duodenal-to-ileal axis in the small intestine, our knowledge regarding endothelial cell regionalization along the intestine remains limited.
Why is this important?
Gaining insights into endothelial cell zonation along the digestive tract offers a promising avenue for manipulating immune regulation, intestinal regeneration and nutrient and drug absorption in a region specific manner. In the long term, the expression of transcriptional regulators responsible for maintaining regional identity in generic endothelium will facilitate the development of region-specific vascularized intestinal tissue.
How do we address this?
By employing single cell and spatial transcriptomic approaches, mouse genetic models, and human tissue, we explore how regional endothelial cell identity is established during development and how it is influenced by microbial content, dietary variations, or injury.
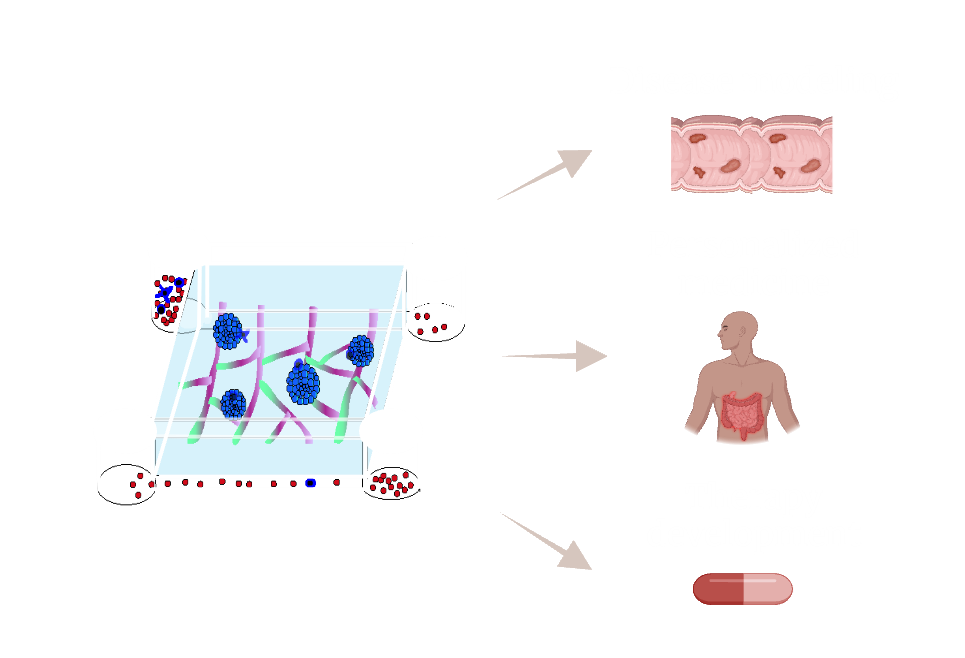
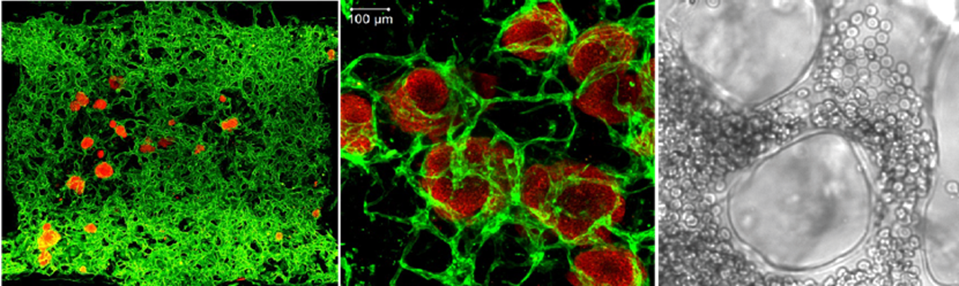
Establishing complex three-dimensional vascularized intestinal tissue
Organoid platforms have become invaluable tools in biomedical research, with wide-ranging applications in tissue transplantation, disease-specific drug screening, and personalized medicine. A key challenge in advancing these platforms is improving their physiological relevance, particularly by aligning their cell composition more closely with in vivo tissues. One promising approach is the integration of vascular networks into organoids, allowing for the development of in vitro models that more accurately replicate the complexities of living tissues. However, a major obstacle in engineering vascularized tissues is the instability of current vascular networks, often requiring bioprinted, pre-patterned scaffolds that limit their dynamic interactions with organoids. In our lab, we aim to overcome this challenge by leveraging the ETV2-endothelial cell platform, which enables the self-organization of stable, extensive vascular networks without the need for pre-patterned structures. We will use this platform to engineer vascularized organoids derived from both healthy individuals and patients with inflammatory bowel disease (IBD). Given the diversity in transcriptional signatures and functional characteristics of endothelial cells across different tissues, our work will pioneer the transition from generic endothelial models to vascular organoids featuring intestine- and colon-specific endothelium.
Why is this important?
Our research aims to create complex and physiologically relevant vascularized organoid systems, with tissue specific endothelium. These platforms can serve as invaluable tools for in vitro disease modeling, drug screening, and hold the potential for future transplantation to replace damaged tissue.
How do we address this?
How do we address this?
We will introduce tissue specific transcription factors in generic endothelium and leverage the ETV2-endothelial cell platform, to achieve the vascularization of human organoids with intestine- and colon-specific endothelium.


